UNIT 1
1.1.1 Charging by rubbing/ conduction/friction
Activity 1 1. Cuts a sheet of toilet paper in to small pieces.
2. Bring a ruler towards the pieces of paper. what happens?
3. Rub the length of the ruler with a cloth.
4. Bring a ruler towards the pieces of paper again, what happens this time?
Explanation. Both a ruler and a piece of cloth were neutral (number of positives equal number of negatives) at the start of the activity.
During rubbing there was a transfer of electrons from one object to another between the two object that were rubbing.
One object had gained electrons (negatively charged) while the other lost electrons (positively charged).
In the end both objects( a ruler and a cloth) that were rubbing were both charged up. as shown in figure 1.1
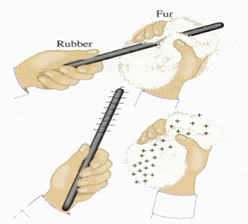
Charging by friction
The presence of different atoms in objects provides different objects with different electrical properties. One such property is known as electron affinity. Simply put, the property of electron affinity refers to the relative amount of love that a material has for electrons. If atoms of a material have a high electron affinity, then that material will have a relatively high love for electrons. This property of electron affinity will be of utmost importance as we explore one of the most common methods of charging - charging by friction or rubbing.
Suppose that a rubber rod is rubbed with a sample of animal fur. During the rubbing process, the atoms of the rubber are forced into close proximity with the atoms of the animal fur. The electron clouds of the two types of atoms are pressed together and are brought closer to the nuclei of the other atoms. The protons in the atoms of one material begin to interact with the electrons present on the other material. Amidst the sound of crackling air, you might even be able to hear the atoms saying, "I like your electrons." And of course, the atoms of one material - in this case, the atoms of rubber - are more serious about their claim for electrons. As such, the atoms of rubber begin to take electrons from the atoms of animal fur. When the rubbing has ceased, the two objects have become charged.